Wilkinson catalyst, commonly represented as RhCl(PPh3)3, is one of the most well-known and widely used catalysts in organic chemistry, particularly in hydrogenation reactions. Developed by Sir Michael Wilkinson in the 1960s, it has since played a crucial role in both academic research and industrial applications, particularly due to its high efficiency in hydrogenating alkenes and other unsaturated compounds under mild conditions. This article explores the purification methods of Wilkinson catalyst, its mechanism, and the importance of the RhCl(PPh3)3 complex in catalysis.
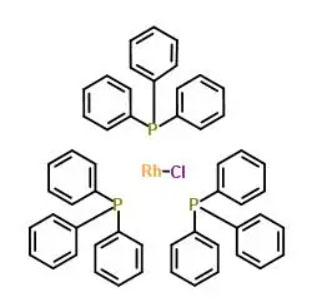
Wilkinson catalyst is a rhodium (Rh)-based coordination complex that consists of two molecules of triphenylphosphine (PPh₃) and a rhodium ion, coordinated to two chloride (Cl) ligands. Its chemical formula is commonly written as RhCl(PPh3)3. The catalyst is typically synthesized through a reaction between rhodium chloride and triphenylphosphine in an appropriate solvent, followed by crystallization and purification. The rhodium center in the catalyst is in a +1 oxidation state, which is crucial for its catalytic activity.
Wilkinson catalyst is especially well-known for its role in hydrogenation reactions, where it facilitates the addition of hydrogen to unsaturated compounds such as alkenes. This makes it an essential catalyst in various chemical processes, including the production of fine chemicals, pharmaceuticals, and petrochemicals.
The catalytic cycle of Wilkinson catalyst is a well-understood process that involves several steps, typically including coordination, activation, and reductive elimination:
Step 1: Coordination of the Substrate The reaction begins when the unsaturated substrate, such as an alkene, coordinates to the rhodium center. The electron-rich double bond of the alkene interacts with the rhodium center, displacing one of the triphenylphosphine ligands. This coordination activates the substrate, preparing it for hydrogenation.
Step 2: Hydrogenation In the presence of molecular hydrogen (H₂), the rhodium-hydride species (RhCl(PPh3)3) forms. The hydrogen atom is transferred to the coordinated alkene, forming a rhodium-alkene-hydride intermediate.
Step 3: Reductive Elimination The final step involves the reductive elimination of the hydrogenated product from the rhodium center. The catalyst regenerates its active form, and the hydrogenated product is released. The cycle then repeats with new substrates and hydrogen molecules.
The ability of Wilkinson catalyst to selectively hydrogenate alkenes without affecting other functional groups, like carbonyls or nitro groups, makes it highly useful in organic synthesis. Its mild conditions and high selectivity are particularly beneficial for sensitive substrates.
Although Wilkinson catalyst is highly efficient, its purity is essential for ensuring optimal catalytic activity. Purifying the catalyst involves several techniques that can remove impurities or residual reaction byproducts. These purification methods are crucial both for laboratory-scale reactions and large-scale industrial processes. Some common purification techniques for Wilkinson’s catalyst include:
Recrystallization: One of the most straightforward methods for purifying Wilkinson catalyst is recrystallization. The catalyst is dissolved in a suitable solvent such as chloroform or dichloromethane. Afterward, slow evaporation or cooling induces the formation of pure crystals of RhCl(PPh3)3. The crystallization process separates the pure catalyst from soluble impurities or unreacted starting materials.
Column Chromatography: For further purification, column chromatography can be employed. The catalyst is placed on a chromatographic column packed with silica gel, and various solvents are used to elute the components. This method is especially useful when the catalyst has multiple impurities or is synthesized with residual ligands.
Washing: In some cases, simple washing with appropriate solvents can remove residual reactants and soluble byproducts. After washing, the catalyst is typically dried under reduced pressure to ensure the removal of all solvents.
These methods help ensure that Wilkinson catalyst is free from contaminants, which could otherwise deactivate the catalyst or affect the efficiency of the hydrogenation reaction.
The primary application of Wilkinson catalyst lies in its ability to catalyze selective hydrogenation reactions.
Selective Hydrogenation of Alkenes: Wilkinson's catalyst can selectively hydrogenate alkenes, converting them into alkanes without affecting other functional groups. This is particularly useful in the pharmaceutical industry, where selective hydrogenation is often required to synthesize complex molecules.
Asymmetric Hydrogenation: In combination with chiral ligands, Wilkinson's catalyst can be used in asymmetric hydrogenation reactions, producing optically active compounds that are important for the production of enantiomerically pure drugs.
Hydrogenation of Aromatic Compounds: The catalyst is also used for the hydrogenation of aromatic compounds, where it can selectively reduce certain double bonds while leaving others untouched.
Wilkinson catalyst (rhcl pph3 3) is a critical reagent in modern organic synthesis, particularly in hydrogenation reactions. Its effectiveness and selectivity make it a go-to choice for chemists and researchers in various industries. The catalyst's purification is vital for ensuring its performance, and several methods, including recrystallization, solvent extraction, and chromatography, are available to achieve high purity. As a versatile and highly efficient catalyst, Wilkinson catalyst continues to be an invaluable tool in both academic and industrial chemistry.
Contact: Tony Li
Phone: +86-13263299644
Tel: +86-13263299644
Email: sales@ecoviaet.com
Add: No 3 Youyi Road,Tangshan,Huantai,Zibo,China
We chat
